MAGNESIUM ANODES.-- Magnesium anodes
have an onboard control of protecting current and
depends on the limited current output of the anode. This
have a half-cell potential of about negative 1.5 volts.
type of system requires anode replacement on a fixed
They aren't used in seawater applications because of
schedule (usually every 3 years on naval ships). The
rapid loss of the anode material and overprotection due
system is rugged and simple, requires little or no
to the high driving voltage. Magnesium anodes are used
maintenance, and always protects the ship.
in fresh or brackish water areas where the resistivity of
the electrolyte is relatively high and a higher driving
Types of Sacrificial Anodes
voltage is required to produce the proper amount of
polarizing current.
The sacrificial anodes listed below are discussed in
this section.
IRON ANODES.-- Iron anodes are installed to
increase the presence of iron ions in the water. The
1. zinc
increase of iron ions strengthens the formation of the
2. Aluminum
oxide film produced on copper alloy surfaces.
3. Magnesium
STEEL WASTER PIECES.-- Steel waster pieces
4. Iron
are sleeves of mild steel installed at nonferrous metal
5. Steel waster pieces
junctions to protect sea valves and sea chests.
ZINC ANODES.-- Zinc anodes are@ for anodic
polarization on steel or aluminum surfaces. They have
Uses of Sacrificial Anodes
a half-cell potential of a negative 1.04 volts. Zinc
anodes can be either bolted or welded to the hull.
Welding is the preferred method because the anodes will
Sacrificial anodes are used in small boats,
have a secure electrical and mechanical attachment.
mothballed ships, and submarines. They may be
installed in piping systems, bilge pumps, valves, ballast
ALUMINUM ANODES.-- Aluminum anodes are
tanks, fuel tanks, sewage collection holding tanks
currently being tested and evaluated by the Naval Sea
(CHTs), sonar domes, voids, and stem tubes.
Systems Command (NAVSEASYSCOM).
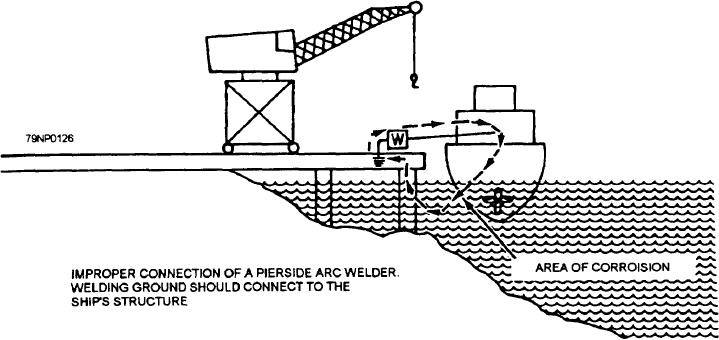
Figure 11-3.--Stray-current corrosion.
11-5

