metal ion in solution goes to the metallic state at a
another half-cell, an overall cell potential develops
cathode in an electrochemical cell.
that is the sum of both half-cell potentials.
Reference electrode-- A half-cell of reproducible
Hydrogen blistering-- The formation of blisterlike
potential by means of which an unknown electrode
bulges on or below the surface of a ductile metal
potential can be determined on some arbitrary scale
caused by excessive internal hydrogen pressure.
(for example, Ag/AgC1, SCE, Cu/CuSO4). A
Hydrogen may be formed during cleaning, plating,
standard against which the potentials of other metal
corrosion, or cathodic protection.
and nonconductive conductor electrodes are
measured and compared.
caused by the presence of hydrogen in the metal; for
example, through pickling, cleaning, of cathodic
Shield-- A nonconducting coating, paint, or sheet that is
protection.
used to beneficially change the current on a cathode
or anode; normally used with impressed current or
Ion-- An electrically charged atom (such as Na+, C+)
other high-potential cathodic protection systems to
or group of atoms (such as NH4+, SO=, PO=).
distribute the current beyond the immediate vicinity
Noble-- A state in which a metal tends not to be active;
of the electrode.
the positive direction of electrode potential.
Stray-current corrosion-- Corrosion caused by current
Noble metal-- A metal that is not very reactive, such as
flow from a source (usually dc) through paths other
silver, gold, or platinum, and that may be found
than the intended circuit or by extraneous currents
naturally in metallic form on earth.
in the electrolytic solution.
Noble potential-- Apotential toward the positive end of
a scale of electrode potentials.
CATHODIC PROTECTION
Open-circuit potential-- The potential of an electrode
measured with respect to a reference electrode when
Cathodic protection reduces the corrosion or
essentially no current flows to or from the electrode.
deterioration of metal caused by a reaction with its
Oxidation-- Loss of electrons by a metal during a
environment (ship's hull and seawater). The chemical
chemical or electrochemical reaction; as when a
action that is created is similar to the electrochemical
metal goes from the metallic state to the corroded
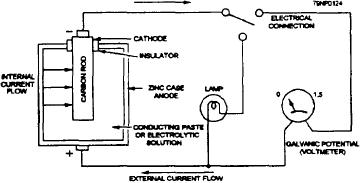
action of a battery or cell. Figure 11-1 shows a dry-cell
state when acting as an anode; when a metal reacts
battery circuit. The positive current is indicated by a
with oxygen sulfix, and so on to form a compound
positive deflection of the voltmeter needle when the
such as oxide or sulfide.
positive terminal of the meter is connected to the
cathode (positive terminal) of the cell. As the
pH-- A logarithmic measure of the acidity or akalinity
electrochemical action continues, the process will
of a solution. A value of 7 is neutral; low numbers
eventually corrode or consume, the anode that is
are acid (1-6); large numbers are alkaline (8-14).
providing the current to light the lamp. This process is
Each unit represents a tenfold change in
called electrochemical action.
concentration.
Polarization-- The shift in electrode potential from the
open-circuit potential value resulting from the
effects of current flow.
Potential-- A numerical value (measured in volts) for
an electrode in a solution and defined with reference
to another specified electrode.
Protective potential-- A term used in cathodic
protection to define the minimum potential required
to mitigate or suppress corrosion. For steel in
quiescent seawater a value of -0.80 volt to Ag/AgC1
reference electrode is generally used.
Reduction-- Gain of electrons by a metal during a
Figure 11-1.--Dry-cell battery circuit.
chemical or electrochemical reaction; as when a
11-3

