ELECTROCHEMICALACTION
between two dissimilar metals. Generally, normal
seawater has a nominal resistivity of 20 to 22 Ohms/cm
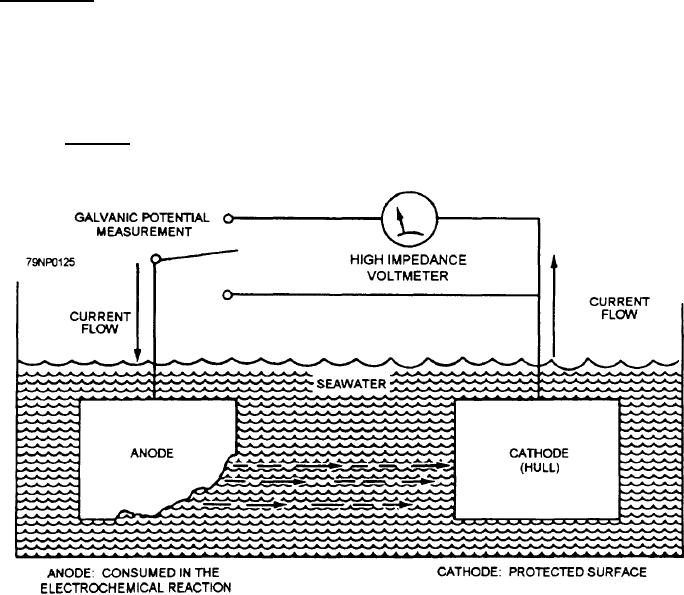
In a marine environment, corrosion is an
at a temperature of 20C (68F). In brackish or fresh
electrochemical process caused when two dissimilar
water this resistivity may vary.
metals are immersed in seawater, with the seawater
acting as the electrolyte. This process is shown in the
TYPES OF CATHODIC
electrochemical corrosion cell (fig. 11-2). You must
PROTECTION
understand that in an electrochemical cell, a metal that
is more corrosion prone always has a higher driving
There are two types of cathodic protection
voltage than the metal that is less corrosion prone. In
systems--the sacrificial anode system and the
impressed current system. Each system will be
cathodic protection the more corrosion-prone metal is
the anode (zinc, for example) and the less
addressed separately.
corrosion-prone metal is the cathode (steel hull, for
example). The rate of corrosion is directly related to the
SACRIFICIAL ANODE SYSTEM
magnitude of the potential difference and is referred to
as the open-or half-cell potential of metals. Some of the
The sacrificial anode system is based on the
factors affecting the amount of corrosion are stray
following principle:
currents, resistivity, and the temperature of the seawater.
When a more reactive metal is installed near a
Stray-current corrosion is caused by an external
less reactive metal and submerged in an
current leaving the hull of a vessel and entering the
electrolyte such as seawater, the more reactive
seawater. If the connection between the ship and
metal will generate a potential of a sufficient
welding machine is not correctly made (fig. 11-3) or no
magnitude to protect the less reactive metal.
return lead to the welder is connected, you could have
In this process, the more reactive metal is sacrificed.
current flow between the ship's hull and the pier. This
Sacrificial anodes attached to a ship's hull slowly
current flow causes corrosion to form on the hull.
oxidize and generate a current (see the electrochemical
Seawater resistivity is the concentration of ions in
corrosion cell in figure 11-2 that protects the hull and its
seawater, which acts as a resistance to current flow
appendages). The system shown in figure 11-2 does not
Figure 11-2.-Electrochemical corrosion cell.
11-4

