are mils per year (mpy), millimeters per year
(Should not be used to mean corrosion by stray
currents.)
(mm/y), and micrometers per year (m/y). 1 mil=
0.001 inch 1 mm= 0.001 meter, 1 m = 0.000001
Electrolyte-- A substance that, in solution, gives rise to
meter.
ions; an ionic conductor usually in aqueous
solution a chemical substance which on dissolving
Current capacity-- The hours of current that can be
in water renders the water conductive.
obtained from a unit weight of a galvanic anode
metal. Usually expressed in ampere hours per
Electrolytic cell-- A system in which an anode and
pound (amp-hr/lb) or ampere hours per kilogram
cathode are immersed in an electrolytic solution and
(amp-h/kg).
electrical energy is used to bring about electrode
reactions. The electrical energy is thus converted
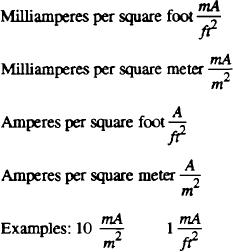
Current density-- The current per unit area of surface of
into chemical energy. ( N O T E : The term
an electrode. Commonly used units include the
electrochemical cell is frequently used to describe
following:
both the electrochemical cell and the electrolytic
cell.)
Electrolytic solution-- A solution that conducts electric
current by the movement of ions.
Electromotive force (emf) series-- A list of elements
arranged according to their standard electrode
potentials (the hydrogen electrode is a reference
point and is given the value of zero), with noble
metals, such as gold being positive, and active
metals, such as zinc, being negative.
Embrittlement-- Severe loss of ductility of a metal or an
alloy.
Current efficiency-- The ratio of the actual total current
External circuit-- Wires, connectors, measuring
measured from a galvanic anode in a given time
devices, current sources, and so forth, that are used
period to the total current calculated from the weight
to bring about or measure the desired electrical
loss of the anode and the electrochemical equivalent
conditions within the test cell. In corrosion
of the anode metal is expressed as a percentage.
terminology it also includes that part of an
electrochemical cell external to the solution
Eletrochemical cell-- A system consisting of an anode
and a cathode immersed in an electrolytic solution.
Galvanic corrosion-- Corrosion of a metal because of
The anode and cathode may be different metals or
electrical contact with a more noble metal or
dissimilar areas on the same metal surface. A cell
nonmetallic conductor in a corrosive environment.
in which chemical energy is converted into
Often used to refer specifically to bimetallic
electrical energy under the condition of current flow
corrosion; sometimes called couple action.
between anode and cathode.
Galvanic couple-- Two or more dissimilar connductors,
Electrode-- A metal or nonmetallic conductor in contact
commonly metals, in electrical contact in the same
with an electrolytic solution that serves as a site
electrolytic solution.
where an electric current enters the metal or
nonmetallic conductor or leaves the metal or
Galvanic series-- A list of metals and alloys arranged
nonmetallic conductor to enter the solution.
according to their relative corrosion potentials in a
given environment. (NOTE: This series may not
Electrode potential-- The difference in electrical
be the same order as in the EMF series.)
potential between an electrode and the electrolytic
solution with which it is in contact; measured
Half-cell-- One of the electrodes and its immediate
relative to a reference electrode.
environment in an electrochemical cell; an
electrode and environment arranged for the passage
Electrolysis-- The production of chemical changes in an
of current to another electrode for the measurement
electrolytic solution caused by the passage of
of its electrode potential; when coupled with
electrical current through an electrochemical cell.
11-2

